ISSUE1698
- Mark Abramowicz, M.D., President has disclosed no relevant financial relationships.
- Jean-Marie Pflomm, Pharm.D., Editor in Chief has disclosed no relevant financial relationships.
- Michael Viscusi, Pharm.D., Associate Editor has disclosed no relevant financial relationships.
- Discuss the appropriate use of the naloxone products available for sale over the counter for reversal of opioid overdose.
The FDA has approved RiVive (Harm Reduction Therapeutics), a 3-mg naloxone nasal spray, as an over-the-counter (OTC) product for emergency treatment of opioid overdose.1 Two 4-mg naloxone nasal spray formulations, Narcan and one of its generics, were approved for OTC sale in 2023.1,2
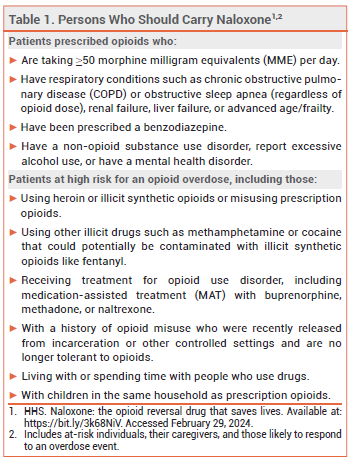
NALOXONE — Naloxone is the drug of choice for reversal of opioid overdose. Every state in the US now has a naloxone access law; in most states, these laws grant both civil and criminal immunity to laypersons who administer the drug.3 The US Department of Health and Human Services has recommended that certain individuals who are prescribed opioids or are at high risk for an opioid overdose, their caregivers, and other persons who are likely to respond to an overdose event carry naloxone nasal spray (see Table 1).4
CLINICAL STUDIES — No new clinical trials were required for approval of RiVive. Approval was based on pharmacokinetic data (summarized in the FDA review documents) showing that systemic naloxone exposure is about 3-fold higher and early naloxone absorption is similar with a 3-mg intranasal dose of the new formulation compared to a 0.4-mg IM dose of naloxone.1,5 RiVive has not been compared directly with other naloxone nasal sprays.
DOSAGE, ADMINISTRATION, AND COST — RiVive is supplied in packages containing two single-use nasal spray devices, each of which delivers 3 mg of naloxone. The recommended initial dose is one spray in one nostril; if necessary, additional doses can be administered every 2-3 minutes until emergency medical personnel arrive.
RiVive is being marketed primarily to US harm reduction organizations and government entities, many of which provide intranasal naloxone to residents at no cost.6,7 A box containing two doses costs $36,8 which is less than the OTC cost of two doses of Narcan ($45) or its generic ($40).9
- FDA News Release. FDA approves second over-the-counter naloxone nasal spray product. July 28, 2023. Available at: https://bit.ly/49BGlxq. Accessed February 29, 2024.
- In brief: Over-the-counter Narcan nasal spray. Med Lett Drugs Ther 2023; 65:72.
- Legislative Analysis and Public Policy Association. Naloxone: summary of state laws. July 2022. Available at: https://bit.ly/3ZNK1H9. Accessed February 29, 2024.
- HHS. Naloxone: the opioid reversal drug that saves lives. Available at: https://bit.ly/3k68NiV. Accessed February 29, 2024.
- Center for Drug Evaluation and Research. Integrated review. RiVive (naloxone hydrochloride) 3 mg/0.1 mL nasal spray. July 21, 2023. Available at: https://bit.ly/4bVG0I8. Accessed February 29, 2024.
- NEXT Distro. Available at: https://nextdistro.org. Accessed February 29, 2024.
- National Harm Reduction Coalition. Find harm reduction resources near you. Available at: https://bit.ly/49JceEI. Accessed February 29, 2024.
- Cost according to the manufacturer.
- Cost at www.walgreens.com. Accessed February 29, 2024.