ISSUE1746
- Jean-Marie Pflomm, Pharm.D., Editor in Chief has disclosed no relevant financial relationships.
- Brinda M. Shah, Pharm.D., Consulting Editor has disclosed no relevant financial relationships.
- Review the efficacy and safety of sublingual cyclobenzaprine (Tonmya) for treatment of fibromyalgia.
- Description: A sublingual formulation of the skeletal muscle relaxant cyclobenzaprine.
- Indication: Treatment of fibromyalgia.
- Efficacy: In three randomized trials in patients with fibromyalgia, reductions in pain intensity scores were significantly greater with sublingual cyclobenzaprine than with placebo in two trials but not in the third.
- Adverse Effects: Common adverse effects included oral hypoesthesia and paresthesia, abnormal taste, and somnolence.
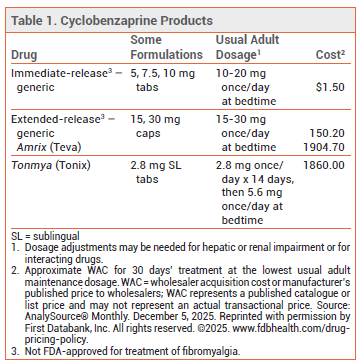
- Dosage: 2.8 mg once daily for 14 days, then 5.6 mg once daily. The drug should be taken at bedtime.
- Cost: A 30-day supply costs $1860.
- Conclusion: Sublingual cyclobenzaprine modestly reduced pain intensity and improved sleep quality in patients with fibromyalgia. How it compares to oral cyclobenzaprine or other drugs used for this indication remains to be determined.
Table
The FDA has approved Tonmya (Tonix), a sublingual tablet formulation of the skeletal muscle relaxant cyclobenzaprine, for treatment of fibromyalgia in adults. Cyclobenzaprine is available in immediate-release tablets and extended-release capsules for short-term treatment of muscle spasms and has been used off-label for treatment of fibromyalgia.

THE DISORDER — Fibromyalgia is a chronic generalized musculoskeletal pain disorder characterized by sleep disturbances, fatigue, cognitive dysfunction, and depression.
STANDARD TREATMENT — A tricyclic antidepressant (TCA) such as amitriptyline, a serotonin-norepinephrine reuptake inhibitor (SNRI) such as duloxetine (Cymbalta, and generics), milnacipran (Savella) or venlafaxine (Effexor XR, and generics), or a gabapentinoid such as pregabalin (Lyrica, and generics) or gabapentin (Neurontin, and others) is generally used for initial pharmacologic treatment of fibromyalgia, but only duloxetine, milnacipran, and immediate-release pregabalin have been approved by the FDA for this indication. All of these drugs have been shown to improve pain, but not sleep quality or fatigue, and their use has been limited by adverse effects.1,2 Oral cyclobenzaprine has improved pain and sleep quality, but its sedative effects can persist the following day.3
PHARMACOLOGY — Cyclobenzaprine is structurally similar to TCAs. Its mechanism of action for treatment of fibromyalgia is unclear. Sublingual administration of cyclobenzaprine bypasses first-pass hepatic metabolism, resulting in reduced exposure to the active metabolite norcyclobenzaprine and fewer anticholinergic adverse effects than oral immediate-release cyclobenzaprine. Sublingual cyclobenzaprine has a faster onset of action but a shorter duration of action than the oral formulations.
CLINICAL STUDIES — FDA approval of sublingual cyclobenzaprine was based on the results of three double-blind trials (RELIEF and RESILIENT have been published; the third trial is summarized in the package insert) in a total of 1474 patients 18-65 years old with fibromyalgia. Patients were randomized to receive sublingual cyclobenzaprine (2.8 mg for 14 days, followed by 5.6 mg for 12 weeks) or placebo once daily. In the RELIEF and RESILIENT trials, the mean change from baseline to week 14 in the weekly average of daily pain intensity scores (on a 0-10 scale, with higher scores indicating more severe pain) was statistically significantly greater with cyclobenzaprine than with placebo (-1.91 vs -1.51 in RELIEF and -1.8 vs -1.2 in RESILIENT). Improvements in sleep quality were also observed.4,5 In the third trial, there was no significant difference in the weekly average of daily pain intensity score between the cyclobenzaprine and placebo arms.
No trials directly comparing sublingual cyclobenzaprine with oral cyclobenzaprine or other drugs used for treatment of fibromyalgia are available.
ADVERSE EFFECTS — Common adverse effects of cyclobenzaprine in the RELIEF and RESILIENT trials included oral hypoesthesia and paresthesia, abnormal taste, and somnolence. Cyclobenzaprine can cause arrhythmias, tachycardia, and CNS depression. Because of its anticholinergic effects, the drug should be used with caution in patients with urinary retention, angle-closure glaucoma, or increased intraocular pressure.
DRUG INTERACTIONS — Use of cyclobenzaprine within 14 days of a monoamine oxidase (MAO) inhibitor is contraindicated. Coadministration of cyclobenzaprine and other serotonergic drugs (e.g., SSRIs, SNRIs, TCAs, MAO inhibitors) can cause serotonin syndrome and is not recommended. Use of cyclobenzaprine with other CNS depressants such as alcohol can result in additive effects. Concurrent use of tramadol and cyclobenzaprine can increase the risk of seizures because both drugs lower the seizure threshold.
PREGNANCY AND LACTATION — In studies in pregnant animals, administration of oral cyclobenzaprine during embryogenesis was associated with neural tube defects and decreased pup survival. Use of cyclobenzaprine during the first trimester of pregnancy should be avoided. Women should use effective contraception while taking the drug and for 2 weeks after the last dose. Cyclobenzaprine has been detected in low levels in human milk.
DOSAGE AND ADMINISTRATION — The recommended dosage of Tonmya is 2.8 mg once daily at bedtime for 14 days, followed by 5.6 mg once daily at bedtime. Older patients and those with mild hepatic impairment should remain on the 2.8-mg dose. Taking the drug at bedtime is intended to improve sleep quality and minimize daytime anticholinergic effects. The tablets should be allowed to dissolve under the tongue; they should not be swallowed whole, cut, or chewed. Patients should avoid eating or drinking for at least 15 minutes after taking the drug and should not consume hot, cold, or acidic beverages until the next morning. Use of the drug is not recommended in patients with moderate or severe hepatic impairment (Child-Pugh B or C) and is contraindicated for use in those who had a recent myocardial infarction or who have arrhythmias, heart block, conduction disturbances, or heart failure.
CONCLUSION — The sublingual formulation of cyclobenzaprine (Tonmya) can modestly reduce pain intensity and improve sleep quality in patients with fibromyalgia. Direct comparisons with less expensive oral cyclobenzaprine formulations or other drugs used for this indication are lacking.
- EA Jones et al. Management of fibromyalgia: an update. Biomedicines 2024; 12:1266. doi:10.3390/ biomedicines12061266
- Nonopioid drugs for pain. Med Lett Drugs Ther 2022; 64:33.
- H Moldofsky et al. Effects of bedtime very low dose cyclobenzaprine on symptoms and sleep physiology in patients with fibromyalgia syndrome: a double-blind randomized placebo-controlled study. J Rheumatol 2011; 38:2653. doi:10.3899/jrheum.110194
- S Lederman et al. Efficacy and safety of sublingual cyclobenzaprine for the treatment of fibromyalgia: results from a randomized, double-blind, placebo-controlled trial. Arthritis Care Res (Hoboken) 2023; 75:2359. doi:10.1002/acr.25142
- S Lederman et al. Pain relief by targeting nonrestorative sleep in fibromyalgia: a phase 3 randomized trial of bedtime sublingual cyclobenzaprine. Pain Med 2025 July 8 (epub). doi:10.1093/pm/pnaf089
The Medical Letter, Inc. does not warrant that all the material in this publication is accurate and complete in every respect. The Medical Letter, Inc. and its editors shall not be held responsible for any damage resulting from any error, inaccuracy, or omission.