ISSUE1744
- Mark Abramowicz, M.D., President has disclosed no relevant financial relationships.
- Jean-Marie Pflomm, Pharm.D., Editor in Chief has disclosed no relevant financial relationships.
- Amy Faucard, MLS, Associate Editor has disclosed no relevant financial relationships.
- Review the efficacy and safety of elinzanetant (Lynkuet) for treatment of moderate to severe vasomotor symptoms due to menopause.
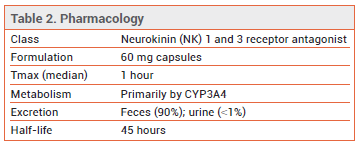
- Description: An oral neurokinin (NK) 1 and 3 receptor antagonist.
- Indication: Treatment of moderate to severe vasomotor symptoms (VMS) due to menopause.
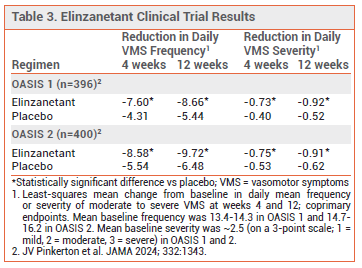
- Efficacy: In two double-blind trials in postmenopausal women with ≥50 moderate to severe VMS per week, elinzanetant significantly reduced the frequency and severity of VMS compared to placebo.
- Adverse Effects: CNS adverse effects are common. Headache, abdominal pain, rash, diarrhea, and muscle spasms can occur.
- Drug Interactions: Use with strong CYP3A4 inhibitors or grapefruit juice, with strong or moderate CYP3A4 inducers, or with sensitive CYP3A4 substrates should be avoided. Dosage should be reduced when used with moderate CYP3A4 inhibitors.
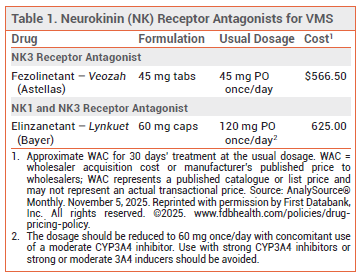
- Dosage: 120 mg once daily at bedtime.
- Cost: A 30-day supply costs $625.
- Conclusion: Elinzanetant is an effective nonhormonal alternative to systemic estrogen for treatment of menopausal VMS. It appears to be more effective than fezolinetant (Veozah) in improving sleep quality.
Tables
Elinzanetant (Lynkuet – Bayer), a first-in-class neurokinin 1 (NK1) and neurokinin 3 (NK3) receptor antagonist, has been approved by the FDA for treatment of moderate to severe vasomotor symptoms (VMS) due to menopause. Fezolinetant (Veozah), an NK3 receptor antagonist, was approved for the same indication in 2023.1

TREATMENT OF VASOMOTOR SYMPTOMS ― Systemic estrogen is the most effective treatment for VMS; it has reduced the frequency and severity of hot flashes by 75%. Transdermal estrogen formulations are as effective as oral formulations and are generally considered less likely to cause thromboembolic and other systemic adverse effects. Women with an intact uterus who use systemic estrogen should also receive a progestogen or the selective estrogen reuptake modulator bazedoxifene, available in combination with conjugated estrogens as Duavee, for endometrial protection.
Several nonhormonal drugs are available for treatment of VMS in women who cannot or choose not to take systemic estrogen. In placebo-controlled trials, fezolinetant reduced the daily frequency (by 56-61%) and severity of VMS. A low-dose formulation of the selective serotonin reuptake inhibitor (SSRI) paroxetine (Brisdelle, and generics) that is FDA-approved for treatment of VMS has also reduced the frequency (by 6-10 per week) and severity of hot flashes. Other SSRIs, serotonin-norepinephrine reuptake inhibitors (SNRIs), gabapentinoids, and the anticholinergic drug oxybutynin have been used off-label for treatment of VMS. Complementary and alternative therapies, such as phytoestrogens and black cohosh, are widely used, but high-quality safety and efficacy data are lacking.2
MECHANISM OF ACTION ― Kisspeptin/neurokinin B/dynorphin (KNDy) neurons located in the thermo-regulatory center in the hypothalamus express NK1 and NK3 receptors and their respective ligands, substance P (SP) and neurokinin B (NKB). The decline in estrogen levels during menopause leads to hypertrophy and hyperactivity of KNDy neurons and overexpression of SP and NKB, causing thermoregulatory disruption that results in VMS. SP and NK1 receptors may also have a role in primary insomnia. Elinzanetant binds to NK1 and NK3 receptors, blocking SP and NKB activity.3
CLINICAL STUDIES ― FDA approval of elinzanetant was based on the results of two double-blind trials (OASIS 1 and 2) in a total of 796 postmenopausal women (mean age 54.4-54.8 years) who were experiencing ≥50 moderate to severe hot flashes per week. Patients were randomized to elinzanetant 120 mg once daily for 26 weeks or placebo for 12 weeks followed by elinzanetant for 14 weeks. In both trials, elinzanetant significantly reduced the daily frequency and severity of VMS at weeks 4 and 12, the primary endpoints, compared to placebo (see Table 3). It also significantly improved sleep disturbances and menopause-related quality of life compared to placebo at week 12.4
In another double-blind trial (OASIS 3), 628 postmenopausal women (mean age 54.7 years) with moderate to severe VMS (mean baseline frequency 6.7-6.8 per day; there was no requirement for a minimum number of events per week) were randomized to receive elinzanetant 120 mg or placebo once daily for 52 weeks. The mean change in daily moderate to severe VMS frequency from baseline to week 12 was significantly greater with elinzanetant than with placebo (-5.4 vs -3.5). At week 50, the mean number of moderate to severe VMS events per day was 1.4 with elinzanetant and 3.5 with placebo.3
Two meta-analyses of randomized trials comparing fezolinetant and elinzanetant with placebo for treatment of VMS in menopausal women found that elinzanetant appeared to be slightly more effective than fezolinetant in reducing VMS frequency and severity. It also improved sleep quality compared to fezolinetant.5,6
Use in HR-Positive Breast Cancer – A double-blind trial (OASIS 4) enrolled 473 women 18-70 years old with moderate to severe VMS (mean baseline frequency 11.4-11.5 per day) associated with endocrine therapy (tamoxifen or aromatase inhibitors) for HR-positive breast cancer or its prevention. Patients were randomized to receive elinzanetant 120 mg daily for 52 weeks or placebo for 12 weeks followed by elinzanetant for 40 weeks. The mean change from baseline in daily moderate to severe VMS frequency was significantly greater with elinzanetant than with placebo at week 4 (-6.5 vs -3.0) and week 12 (-7.8 vs -4.2); elinzanetant also significantly decreased sleep disturbances compared to placebo. Among patients who received elinzanetant for up to 52 weeks, the drug was well tolerated and improvements in VMS were maintained. Elinzanetant is not currently approved for this indication.7
ADVERSE EFFECTS ― In the OASIS 1-3 trials, the incidence of CNS adverse effects (including somnolence, fatigue, vertigo, dizziness, and presyncope) was higher with elinzanetant than with placebo (11.9% vs 3.5%). Other common adverse effects of elinzanetant in the 52-week OASIS 3 trial were headache, abdominal pain, rash, diarrhea, and muscle spasms. Mild to moderate photosensitivity occurred rarely. Seizures were observed in animal studies with elinzanetant; in clinical trials, seizure was reported in one patient taking elinzanetant who had a history of seizures.
Transaminase elevations ≥3 times the upper limit of normal (ULN) occurred in 0.6% of patients treated with elinzanetant for up to 12 weeks in clinical trials. Long-term use of elinzanetant in OASIS 3 was not associated with hepatotoxicity. The labeling of fezolinetant contains a boxed warning about a risk of hepatotoxicity based on postmarketing reports of drug-induced liver injury occurring within 40 days of starting the drug.8
DRUG INTERACTIONS ― Elinzanetant is metabolized primarily by CYP3A4; use with strong CYP3A4 inhibitors (e.g., itraconazole) or grapefruit juice or with strong or moderate CYP3A4 inducers should be avoided. The dosage of elinzanetant should be reduced when it is used with a moderate CYP3A4 inhibitor (e.g., erythromycin). Elinzanetant is a weak inhibitor of CYP3A4; use with sensitive CYP3A4 substrates such as midazolam should be avoided.9
DOSAGE AND ADMINISTRATION ― The recommended dosage of elinzanetant is 120 mg (two 60-mg capsules) taken once daily at bedtime. The capsules should be swallowed whole; they should not be cut, crushed, or chewed. A missed dose should be skipped. The dosage should be reduced to 60 mg once daily with concomitant use of a moderate CYP3A4 inhibitor.
Hepatic function should be assessed before starting elinzanetant and 3 months after initiation of therapy. The drug should not be started if alanine aminotransferase (ALT), aspartate aminotransferase (AST), or total bilirubin is ≥2 times the ULN.
CONCLUSION ― The oral neurokinin (NK) 1 and 3 receptor antagonist elinzanetant (Lynkuet) reduces the frequency and severity of moderate to severe vasomotor symptoms (VMS) due to menopause. It has not been compared directly with the NK3 receptor antagonist fezolinetant (Veozah), but indirect comparisons suggest that elinzanetant may be more effective in improving sleep quality. Whether elinzanetant, like fezolinetant, is associated with an increased risk of hepatotoxicity remains to be determined. Both of these drugs are effective nonhormonal options for treatment of VMS in women who cannot or choose not to take systemic estrogen. They are not effective for treatment of other symptoms related to estrogen loss in menopause, such as genitourinary syndrome, depression, and joint pain.
- Fezolinetant (Veozah) for menopausal vasomotor symptoms. Med Lett Drugs Ther 2023; 65:97.
- Drugs for menopausal symptoms. Med Lett Drugs Ther 2024; 66:33.
- N Panay et al. Elinzanetant for the treatment of vasomotor symptoms associated with menopause: a phase 3 randomized clinical trial. JAMA Intern Med 2025; 185:1319. doi:10.1001/jamainternmed.2025.4421
- JV Pinkerton et al. Elinzanetant for the treatment of vasomotor symptoms associated with menopause: OASIS 1 and 2 randomized clinical trials. JAMA 2024; 332:1343. doi:10.1001/jama.2024.14618
- AM de Almeida et al. Fezolinetant and elinzanetant therapy for menopausal women experiencing vasomotor symptoms: a systematic review and meta-analysis. Obstet Gynecol 2025; 145:253. doi:10.1097/aog.0000000000005812
- HM de Oliveira et al. Efficacy and safety of fezolinetant and elinzanetant for vasomotor symptoms in postmenopausal women: a systematic review and meta-analysis. Maturitas 2025; 195:108220. doi:10.1016/j.maturitas.2025.108220
- F Cardoso et al. Elinzanetant for vasomotor symptoms from endocrine therapy for breast cancer. N Engl J Med 2025; 393:753. doi:10.1056/nejmoa2415566
- In brief: New warnings for fezolinetant (Veozah). Med Lett Drugs Ther 2024; 66:168.
- Inhibitors and inducers of CYP enzymes, P-glycoprotein, and other transporters. Med Lett Drugs Ther 2023 January 25 (epub). Available at: www.medicalletter.org/downloads/CYP_PGP_Tables.pdf.
The Medical Letter, Inc. does not warrant that all the material in this publication is accurate and complete in every respect. The Medical Letter, Inc. and its editors shall not be held responsible for any damage resulting from any error, inaccuracy, or omission.