ISSUE1745
- Jean-Marie Pflomm, Pharm.D., Editor in Chief has disclosed no relevant financial relationships.
- Susan Daron, Pharm D., Associate Editor has disclosed no relevant financial relationships.
- Review the efficacy and safety of gepotidacin (Blujepa) for treatment of uncomplicated urinary tract infections.
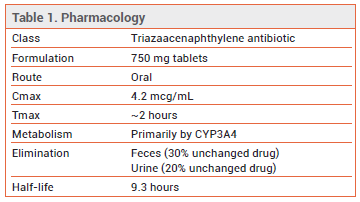
- Description: The first triazaacenaphthylene antibiotic approved in the US.
- Indication: Treatment of uncomplicated urinary tract infection (uUTI) in females ≥12 years old who weigh ≥40 kg.
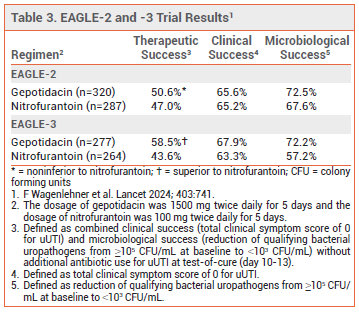
- Efficacy: Gepotidacin was noninferior to nitrofurantoin in one clinical trial and superior to nitrofurantoin in another for treatment of uUTI in nonpregnant female patients.
- Adverse Effects: Most common include diarrhea, nausea, abdominal pain, and flatulence. Can prolong the QT interval and cause cholinergic adverse effects.
- Drug Interactions: Use with CYP3A4 strong inhibitors and CYP3A4 inducers should be avoided. Can increase concentrations of digoxin and drugs that are extensively metabolized by CYP3A4. Use with other drugs that prolong the QT interval or have cholinergic effects can result in additive effects. Can antagonize the effects of anticholinergic drugs.
- Dosage: 1500 mg (two 750-mg tablets) PO every 12 hours after a meal x 5 days.
- Cost: A five-day course costs $1900.
- Conclusion: Gepotidacin should be reserved for use in patients without other treatment options.
Tables
The FDA has approved gepotidacin (Blujepa – GSK), a triazaacenaphthylene bacterial type II topoisomerase inhibitor, for oral treatment of uncomplicated urinary tract infections (uUTI) in female patients ≥12 years old who weigh ≥40 kg. Gepotidacin is the first triazaacenaphthylene antibiotic to be approved in the US.

Gepotidacin has also received approval from the FDA for treatment of uncomplicated urogenital gonorrhea; its use for this indication will be reviewed in a future issue.1
ACUTE uUTI — The most common bacterial cause of acute uUTI in otherwise healthy nonpregnant women is Escherichia coli; other common pathogens include Klebsiella pneumoniae, Proteus mirabilis, and Staphylococcus saprophyticus. Infections caused by drug-resistant E. coli, including extended-spectrum beta-lactamase (ESBL)-producing strains, have been increasing worldwide.2
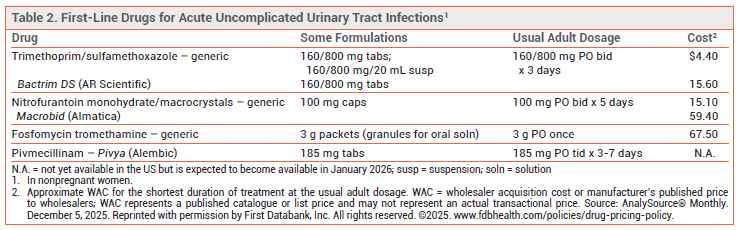
STANDARD TREATMENT — The preferred drugs for first-line empiric treatment of uUTI in nonpregnant women are trimethoprim/sulfamethoxazole, nitrofurantoin, fosfomycin, and pivmecillinam (see Table 2).3-5 Amoxicillin/clavulanate, cefpodoxime, and cefadroxil are second-line options. The fluoroquinolones ciprofloxacin and levofloxacin are alternatives for patients who cannot take a beta-lactam antibacterial drug. Increasing resistance of common uropathogens to all of these drugs is a concern.
Orlynvah, an oral fixed-dose combination of the thiopenem antibacterial drug sulopenem etzadroxil and the renal tubule transport inhibitor probenecid, was recently approved by the FDA for treatment of uUTI.6 It is not recommended for empiric treatment of acute uUTI.
MECHANISM OF ACTION — Gepotidacin blocks bacterial DNA replication by inhibiting two bacterial type II topoisomerases (DNA gyrase and topoisomerase IV).7 Fluoroquinolones also inhibit bacterial type II topoisomerases, but they bind to the enzymes differently.
SPECTRUM OF ACTIVITY — Gepotidacin is active in vitro and in clinical uUTI against the gram-positive organisms Enterococcus faecalis and S. saprophyticus and the gram-negative organisms Citrobacter freundii complex, E. coli, and K. pneumoniae. It has in vitro activity against P. mirabilis.
Unlike with fluoroquinolones, a single target-specific mutation is not expected to significantly impact gepotidacin activity. Gepotidacin has shown in vitro activity against some strains of fluoroquinolone-resistant bacteria, including E. coli.8
CLINICAL STUDIES — FDA-approval of gepotidacin for uUTI was based on the results of two double-blind trials (EAGLE-2 and -3) that randomized nonpregnant females ≥12 years old with uUTI to receive gepotidacin 1500 mg twice daily or nitrofurantoin 100 mg twice daily for 5 days. The most common pathogen in both trials was E. coli (90%). Both studies were stopped early for efficacy after an interim analysis. Among 1148 patients included in the interim analysis set, gepotidacin was noninferior to nitrofurantoin in EAGLE-2 and superior to nitrofurantoin in EAGLE-3 for the primary endpoint of therapeutic success (combined clinical and microbiological success; see Table 3).9
ADVERSE EFFECTS — The most common adverse effects of gepotidacin in the EAGLE trials were diarrhea (16%), nausea (9%), abdominal pain (4%), and flatulence (3%).
QT-interval prolongation has occurred with use of gepotidacin. Use of the drug should be avoided in patients with a history of QT-interval prolongation, in those with relevant pre-existing cardiac disease, and in those taking antiarrhythmics or other drugs that prolong the QT interval. Gepotidacin is a reversible acetylcholinesterase inhibitor; it can cause cholinergic adverse effects such as dysarthria, presyncope, muscle spasms, diarrhea, nausea, vomiting, abdominal pain, hypersalivation, and hyperhidrosis. Increased levels of acetylcholine can also be associated with serious adverse events, including atrioventricular block, bradycardia, bronchospasm, seizures, and syncope.
As with other antibacterial drugs, use of gepotidacin could result in Clostridioides difficile infection.
DRUG INTERACTIONS — Gepotidacin is a substrate of CYP3A4. Use with a strong CYP3A4 inhibitor can increase gepotidacin serum concentrations and its adverse effects, and use with a strong CYP3A4 inducer can decrease gepotidacin serum concentrations and possibly its efficacy. Concurrent use of gepotidacin and strong inhibitors or inducers of CYP3A4 should be avoided.10
Gepotidacin can increase serum concentrations of drugs that are extensively metabolized by CYP3A4; use with such drugs that have a narrow therapeutic index (e.g., quinidine, cyclosporine) should be avoided. Gepotidacin can increase digoxin levels.
Use of gepotidacin with other drugs that prolong the QT interval can result in additive effects and should be avoided. As a reversible acetylcholinesterase inhibitor, gepotidacin may exaggerate the neuromuscular effects of succinylcholine-type anesthetics, may cause additive effects if used in combination with other drugs that have cholinergic effects, and may antagonize the effects of systemic anticholinergic drugs.
PREGNANCY AND LACTATION — No data are available on the use of gepotidacin in pregnant women. Decreased fetal weight and increased fetal mortality were observed in embryofetal development studies in mice and rats at exposures less than the maximum recommended human dose (MRHD). In a pre- and postnatal development study in mice, no adverse developmental effects were observed at exposures approximately equal to the MRHD.
No data are available on the presence of gepotidacin in human milk or its effects on the breastfed infant or milk production. When gepotidacin was administered to lactating mice, it was detected in the plasma of their nursing offspring.
DOSAGE, ADMINISTRATION, AND COST — The recommended dosage of Blujepa for treatment of uUTI is 1500 mg (two 750-mg tablets) taken every 12 hours for 5 days. The dose should be taken after a meal to improve GI tolerability. Use of gepotidacin should be avoided in patients with severe renal (eGFR <30 mL/min/1.73 m2) or hepatic (Child-Pugh C) impairment. The wholesale acquisition cost (WAC) of a 5-day course of Blujepa is $1900.11
CONCLUSION — Gepotidacin (Blujepa), a first-in-class triazaacenaphthylene antibiotic, has been approved by the FDA for treatment of uncomplicated urinary tract infection (uUTI) in female patients ≥12 years old who weigh ≥40 kg. The drug was noninferior to nitrofurantoin for this indication in one clinical trial and superior in another. Gepotidacin can cause QT-interval prolongation and cholinergic adverse effects, has the potential to interact with many other drugs, and is very expensive. It should be reserved for use in patients without other treatment options.
- FDA News Release. FDA approves two oral therapies to treat gonorrhea. December 12, 2025. Available at: https://bit.ly/3MwNOrA. Accessed December 15, 2025.
- PD Tamma et al. Infectious Diseases Society of America 2024 guidance on the treatment of antimicrobial-resistant gram-negative infections. Clin Infect Dis 2024 August 7 (epub). doi:10.1093/cid/ciad428
- K Gupta et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis 2011; 52:e103. doi:10.1093/cid/ciq257
- JY Kikuchi et al. Antibiotic prescribing patterns and guideline concordance for uncomplicated urinary tract infections among adult women in the US military health system. JAMA Netw Open 2022; 5:e2225730. doi:10.1001/jamanetworkopen.2022.25730
- Pivmecillinam (Pivya) – a new antibiotic for UTI. Med Lett Drugs Ther 2025 (in press).
- Sulopenem etzadroxil/probenecid (Orlynvah) for uncomplicated UTIs. Med Lett Drugs Ther 2025; 67:179.
- RR Watkins et al. Gepotidacin: a novel, oral, 'first-in-class' triazaacenaphthylene antibiotic for the treatment of uncomplicated urinary tract infections and urogenital gonorrhoea. J Antimicrob Chemother 2023; 78:1137. doi:10.1093/jac/dkad060
- MA Hackel et al. In vitro activity of gepotidacin against urinary tract infection isolates of Enterobacerales, Enterococcus faecalis, and Staphylococcus saprophyticus. Antimicrob Agents Chemother 2025; 69:e0029625. doi:10.1128/aac.00296-25
- F Wagenlehner et al. Oral gepotidacin versus nitrofurantoin in patients with uncomplicated urinary tract infection (EAGLE-2 and EAGLE-3): two randomised, controlled, double-blind, double-dummy, phase 3, non-inferiority trials. Lancet 2024; 403:741. doi:10.1016/s0140-6736(23)02196-7
- Inhibitors and inducers of CYP enzymes, P-glycoprotein, and other transporters. Med Lett Drugs Ther 2023 January 25 (epub). Available at: www.medicalletter.org/downloads/CYP_PGP_Tables.pdf.
- Approximate WAC. WAC = wholesaler acquisition cost or manufacturer's published price to wholesalers; WAC represents a published catalogue or list price and may not represent an actual transactional price. Source: AnalySource® Monthly. December 5, 2025. Reprinted with permission by First Databank, Inc. All rights reserved. ©2025. www.fdbhealth.com/ drug-pricing-policy.
The Medical Letter, Inc. does not warrant that all the material in this publication is accurate and complete in every respect. The Medical Letter, Inc. and its editors shall not be held responsible for any damage resulting from any error, inaccuracy, or omission.