ISSUE1746
- Jean-Marie Pflomm, Pharm.D., Editor in Chief has disclosed no relevant financial relationships.
- Michael Viscusi, Pharm.D., Associate Editor has disclosed no relevant financial relationships.
- Review the efficacy and safety of the nalmefene autoinjector (Zurnai) for emergency treatment of opioid overdose.
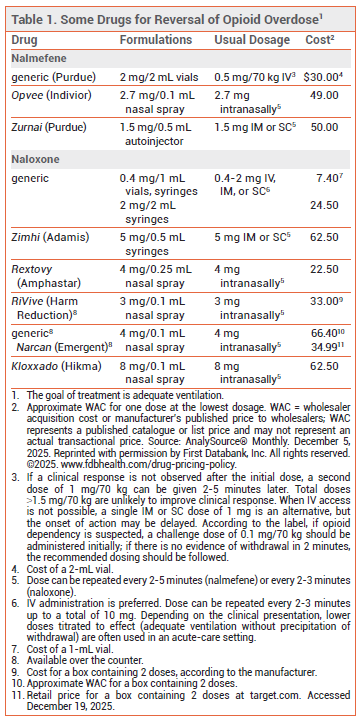
- Description: The first formulation of the opioid antagonist nalmefene approved for intramuscular or subcutaneous injection.
- Indication: Emergency treatment of opioid overdose in persons ≥12 years old.
- Efficacy: In a pharmacodynamic study in opioid users, nalmefene began to reverse respiratory depression within 2.5-5 minutes after injection.
- Adverse Effects: "Feeling hot," chills, nausea, vomiting, dizziness, headache, allodynia, tinnitus, palpitations, and irritability have been reported.
- Dosage: 1.5 mg injected IM/SC, repeated every 2-5 minutes until adequate ventilation is restored or emergency medical personnel arrive.
- Cost: The wholesale cost for one dose is $50.
- Conclusion: Naloxone remains the drug of choice for reversal of opioid overdose.
Table
Zurnai (Purdue), an autoinjector formulation of the opioid antagonist nalmefene, has been approved by the FDA for intramuscular (IM) or subcutaneous (SC) emergency treatment of known or suspected opioid overdose in persons ≥12 years old.1 Naloxone, another opioid antagonist, has been available in single-use syringes for years. Both nalmefene and naloxone are also available in nasal sprays; some naloxone nasal sprays (Narcan, and others) are available over the counter.2,3

NALMEFENE vs NALOXONE — Naloxone has been the drug of choice for emergency treatment of opioid overdose for many years.4 Because the half-life of naloxone (1-2 hours) is shorter than the duration of effect of most opioid agonists, repeat administration of the drug may be needed. In contrast, nalmefene has a longer half-life (~9-11 hours) than most opioid agonists, which could decrease the need for repeat administration, but could cause prolonged withdrawal symptoms in patients who are physically dependent on opioids.5,6
CLINICAL STUDIES — No new clinical efficacy trials were required for FDA approval of Zurnai. In a pharmacodynamic study, 24 opioid-experienced, non-dependent persons were given an IV infusion of fentanyl to induce respiratory depression (~50% reduction in minute ventilation from baseline for 10 minutes), followed by a single 1.5-mg dose of nalmefene via the autoinjector. Reversal of respiratory depression began within 2.5-5 minutes after administration of nalmefene, and full recovery of respiratory drive occurred within 5-15 minutes.7 Clinical data on use of nalmefene for reversal of overdose due to fentanyl or its analogs are lacking.
ADVERSE EFFECTS — The most common adverse effects reported with Zurnai in pharmacologic trials were "feeling hot," chills, nausea, vomiting, dizziness, headache, allodynia, tinnitus, palpitations, and irritability. Severe opioid withdrawal precipitated by nalmefene could result in serious adverse effects, including cardiovascular events and pulmonary edema. In cases of mixed overdose, administration of an opioid antagonist could unmask the effects of other drugs, such as stimulants or nonopioid sedatives.
PREGNANCY — No human data are available on use of nalmefene during pregnancy. Use of high doses of the drug in pregnant rats and rabbits did not result in embryotoxic effects.
DOSAGE AND ADMINISTRATION — Zurnai is available in 1.5 mg/0.5 mL autoinjectors. The recommended dosage is 1.5 mg of nalmefene administered IM or SC (through clothing, if necessary) into the anterolateral aspect of the thigh. According to the manufacturer, additional doses can be given every 2-5 minutes until adequate ventilation is restored or emergency medical personnel arrive.
AVAILABILITY — Unlike naloxone, nalmefene is only available by prescription. Most states have standing orders allowing for distribution of prescription naloxone formulations to first responders and caregivers, but these orders do not always apply to nalmefene.8,9 In most states, access laws that grant civil or criminal immunity to laypersons who administer naloxone appear to also apply to nalmefene.8
CONCLUSION — Zurnai, an autoinjector formulation of the opioid antagonist nalmefene, is now available by prescription for intramuscular or subcutaneous administration to reverse opioid overdose. Nalmefene has a longer duration of action than many opioid analgesics, which could precipitate a prolonged period of withdrawal in patients who are physically dependent on opioids. Naloxone, which is shorter-acting and available for sale over the counter as a nasal spray (Narcan, and others), may be a safer choice.
- FDA News Release. FDA approves first nalmefene hydrochloride auto-injector to reverse opioid overdose. August 7, 2024. Available at: https://bit.ly/3MQuBRy. Accessed December 19, 2025.
- In brief: Over-the-counter Narcan nasal spray. Med Lett Drugs Ther 2023; 65:72.
- In brief: A new OTC naloxone nasal spray (RiVive). Med Lett Drugs Ther 2024; 66:47.
- AI Stolbach et al. American College of Medical Toxicology and the American Academy of Clinical Toxicology position statement: nalmefene should not replace naloxone as the primary opioid antidote at this time. Clin Toxicol (Phila) 2023; 61:952. doi:10.10 80/15563650.2023.2283391
- Drugs for opioid use disorder. Med Lett Drugs Ther 2023; 65:137.
- SC Britch and SL Walsh. Treatment of opioid overdose: current approaches and recent advances. Psychopharmacology (Berl) 2022; 239:2063. doi:10.1007/s00213-022-06125-5
- A Cipriano et al. Reversal of fentanyl-induced respiratory depression in healthy subjects by intramuscular nalmefene administered by auto-injector versus intranasal naloxone. J Clin Pharmacol 2025; 65:1661. doi:10.1002/jcph.70088
- Legislative Analysis and Public Policy Association. Opioid antagonist access: summary of state laws. October 2023. Available at: https://bit.ly/3KHcrBg. Accessed December 19, 2025.
- SAFEProject. State naloxone access rules and resources. Available at: https://bit.ly/4pmT8w4. Accessed December 19, 2025.
The Medical Letter, Inc. does not warrant that all the material in this publication is accurate and complete in every respect. The Medical Letter, Inc. and its editors shall not be held responsible for any damage resulting from any error, inaccuracy, or omission.